By
Shrinwantu Pal
November 2023
Print Version
What you need to know
Discovered in the 1940s, sodium borohydride (NaBH4) is a ubiquitous chemical that is not only available in every chemistry research group but also forms the basis for standard undergraduate organic chemistry experiments. NaBH4 is widely used in chemical reductions and has recently also been exploited as a potential hydrogen storage medium.
Why this research is important
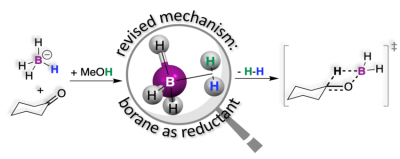
Chemical reductions are an important class of reactions where reducing agents push electrons or other electron-rich fragments into substrates undergoing reduction. One such fragment is hydride (H-). A hydrogen atom (H) is the smallest chemical species imaginable, and therefore, it is no surprise that H- is an electron-rich species. The small size and the large electron density together make H- a very reactive and unstable species. Sodium borohydride (NaBH4) is a molecule that has four such hydrides, which are stabilized by robust chemical bonds to the central B atom in the BH4- anion. The sodium cation serves to make NaBH4 a crystalline solid. These make NaBH4 a bench-stable reducing agent that, pending the right conditions, is ready to transfer H- fragments to substrates such as ketones. However, like other hydrides, the BH4- anion also undergoes reactions with protic solvents (i.e., solvents that can supply protons, H+) such as water or alcohol to form H2 according to the simple equation: H- + H+ → H2. This is a side reaction that serves to deplete the number of hydrides in BH4- available for reduction. Still, surprisingly, reductions with BH4- are performed in alcohols! This is an unsustainable choice that requires excess BH4- to compensate for the loss of H2. On the other hand, reductions performed in aprotic solvents simply do not work! Is it possible that this loss is a necessary evil? This conundrum had not been explained in the 80 years that have passed since the discovery of NaBH4.
How the research was conducted
Firstly, the reaction between the BH4- anion and the protons in solvents like water or alcohol was examined with microscopic precision through density functional theory (DFT) calculations. These calculations indicated that the BH4- anion does not actually transfer hydride fragments as previously thought; rather, it preferentially undergoes a reaction with protons to form an elusive intermediate BH3(H2) that loses H2 to afford the reduction. As a testament to the ability of modern computational methods to predict experimental outcomes, this proposed intermediate was ascertained experimentally by isotope scrambling and nuclear magnetic resonance spectroscopy. A multitude of experimental data from the literature that had not been mechanistically corroborated were assessed and benchmarked against this new mechanism.
What the researcher found
Although reaction mechanisms can never be proven, they can be supported by looking at the rates at which reactions occur, by isotopic substitutions that allow us to track specific atoms in the transformations, or by looking at stereoselectivity, which allows us to compare the geometries of reaction products. For the first time, the intermediacy of BH3(H2) in this work unified two reactions that have remained orthogonal for over 80 years, explaining that the loss of H2 and the need for alcohol as a solvent are actually necessary. This new mechanistic model was benchmarked against these indicators and found to be consistent with experimental rate constants, isotope effects and stereoselectivity data published in over 20 articles since the 1940s.
How this research can be used
The mechanistic insights in this work might promote the exploration of new avenues for reductions of substrates inert to BH4− (such as alkenes, esters, and carboxylic acids). It is anticipated that these mechanistic insights will further our understanding of the reactions of borohydride and its use in hydrogen storage applications.
Acknowledgements
Thank you to Brandon University for a start-up grant and the High-Performance Computing Center at Okinawa Institute of Science and Technology, Japan, for computational resources.
About the Researcher
Keywords
- borohydride
- hydrogen storage
- mechanism
- reduction
Publications Based on the Research
Pal, S. (2023). Protonolysis of BH4– leads to intermediacy of BH3-σ(H2) that evolves H2 and furnishes borane as the key reducing agent. Organometallics, 42(21), 3099–3108. https://doi.org/10.1021/acs.organomet.3c00353
Editor: Christiane Ramsey
Read more BU Research
Research at Brandon University follows comprehensive policies designed to safeguard ethics, to ensure academic integrity, to protect human and animal welfare and to prevent conflicts of interest.

