By
Eric Bushnell and Briana Boychuck
Print Version
What you need to know
We are currently investigating metal-diselenolene for their use for the production of H2(g) to be used in hydrogen fuel cells. Past research in my group has investigated other aspects of the chemistry of such metal-diselenolene complexes and has found subtle differences that make the diselenolene complexes attractive for use in chemical reactions involving the transfer of electrons, such as the production of H2(g) from water.
Why this research is important
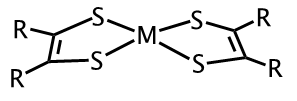
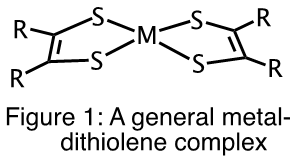
Metal-dithiolene complexes (Figure 1) have been investigated for potential use in the purification of olefins used in the production of polymers, dye-sensitized solar cells, photo catalysts for the splitting of H2O to produce H2 and more recently as photothermal therapeutic agents for the treatment of cancer.
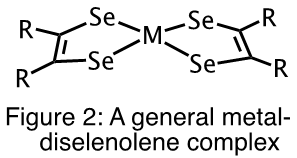
Given the rich and diverse chemistry of metal-dithiolene complexes, it is not surprising that they are well investigated. However, analogous metal-diselenolene complexes (Figure 2) have not been as well studied.

Increasing levels of greenhouse gases in our atmosphere threaten our planet as a result of climate change. A main component of greenhouse gases is carbon dioxide and is a primary product of fossil-fuel consumption which is required for the planet's energy demands. In fact, 80% of the world's energy consumption is derived from the burning of fossil fuels. Based on current emission levels of CO2 into our atmosphere, the Earth is on track for continued global temperature increases. Importantly, it has been stated that an increase of 2 °C in Earth's average temperature is considered a benchmark where the risk of climate change becomes threatening. Based on the current levels of CO2 emissions, the probability of reaching this 2 °C benchmark by 2100 is 88%! Thus, to prevent this increase of 2 °C urgent action is required. Such action involves reduced fossil fuel consumption and the development of renewable energy sources to reduce the amount of CO2 emitted into our atmosphere.
Currently, there are several alternative energy sources available such as wind, solar, and tidal. The most viable path for large-scale growth of alternative energy sources involves solar energy conversion and—specifically—the photocatalytic splitting of water to form molecular hydrogen to be used as an alternative fuel. While hydrogen is the most abundant element in the universe, molecular hydrogen itself is not present on earth in significant amounts and must be produced from a hydrogen-containing source, such as water. Electrolysis of water allows for the conversion of electricity into chemical energy, allowing for separation of hydrogen from water. However, presently there are limited methods available for hydrogen fuel production that are efficient and do so at relatively low costs. As a means to minimize costs, research is under way in the development of various catalysts using cheap-metals such as copper, nickel, iron, and cobalt. However, many catalysts previously investigated for the production of molecular hydrogen decompose over time due to the hydrogenation of the ligands.
How this research was conducted
In the research lab, a computational chemistry tool was used, which involves the use of computers to solve the complicated equations of quantum mechanics allowing chemical systems and reactions to be studied at the atomic level. We studied the Ni-bis (1,2-diamine-diselenolene) and Ni-bis(1,2-dicyano-diselenolene) complexes using Density Functional Theory for the renewable production of H2(g) to be used in hydrogen fuel cells.
What the researchers found
Present results from my research program are promising and suggest that select nickel-diselenolene catalyze the formation of H2(g) with a small energetic cost in a dilute aqueous environment.
How this research can be used
Knowledge gained from this work and ongoing work will provide insight into the design of new catalysts for the renewable production of H2 via the photocatalytic splitting of water. Moreover, the results from my research may offer solutions to the problems currently faced in the production of H2 seen in past experimental work.
Acknowledgements
EACB thanks NSERC for funding. EACB and BTAB thank WESTGRID, SHARCNET and Compute Canada for computational resources.
About the Researchers
Keywords
- alternative fuels
- amphibian
- density functional theory
- diselenolene
- HER catalysts
- hydrogen evolution reaction
- nickel-bis(diselenolene)
Publications Based on the Research
Boychuk, B. T. A., Bushnell, E. A. C. (Forthcoming). A computational investigation into nickel-bis(diselenolene) complexes as potential catalysts for reduction of H+ to H2. Canadian Journal of Chemistry.
Editor: Christiane Ramsey
Read more BU Research
Research at Brandon University follows comprehensive policies designed to safeguard ethics, to ensure academic integrity, to protect human and animal welfare and to prevent conflicts of interest.

